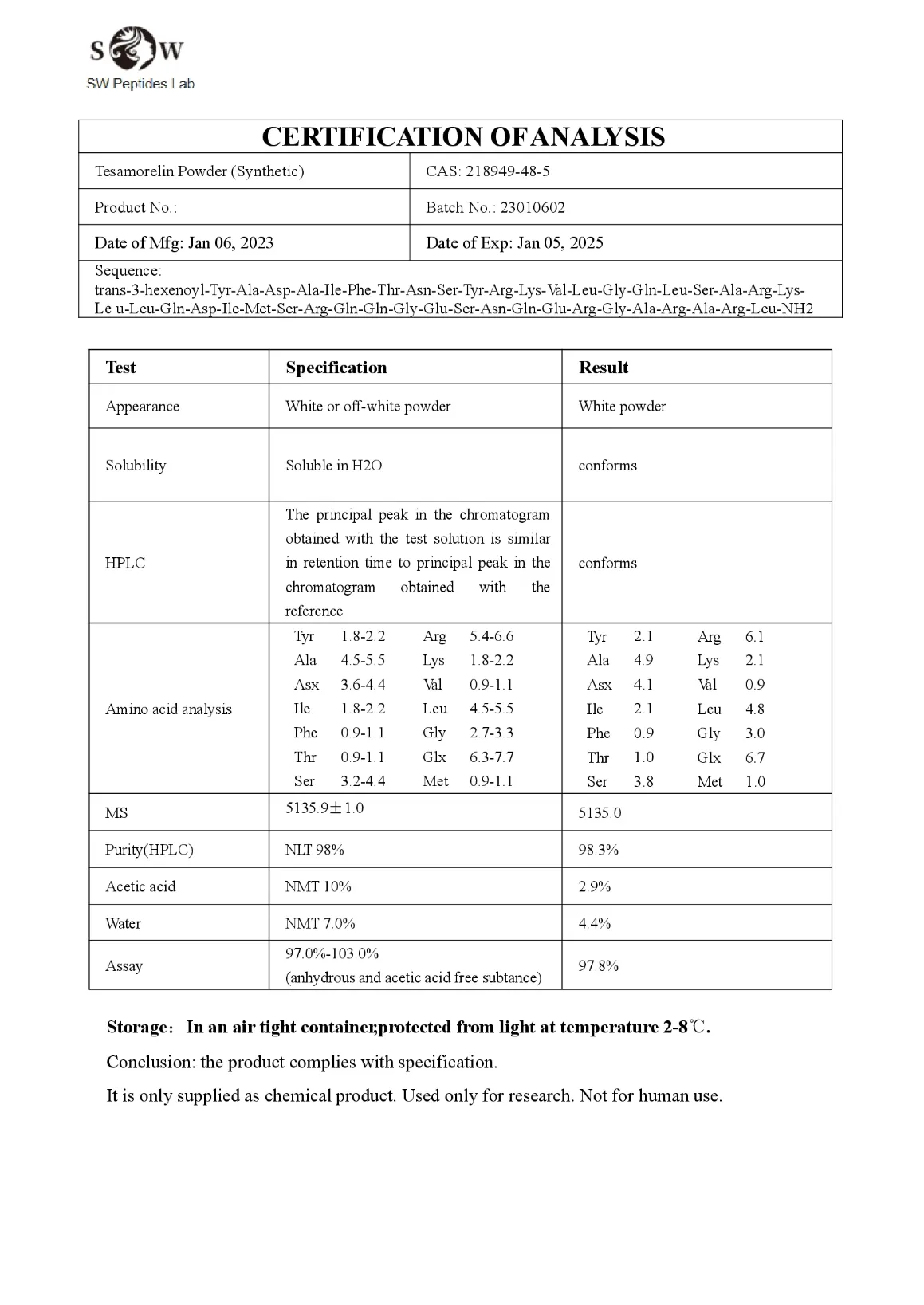
Tesamorelin 2mg
$25.00 - $35.00
You save
- Physical profile: Lyophilized powder
- This product is sold as a research chemical and not for human or animal consumption. For laboratory use by qualified professionals.
Out of stock
Availability: Ships today if ordered and paid by 12 PM EST. (Except Saturdays & Sundays)
Product Usage
This PRODUCT IS INTENDED AS A RESEARCH CHEMICAL ONLY. This designation allows the use of research chemicals strictly for in vitro testing and laboratory experimentation only. All product information available on this website is for educational purposes only. Bodily introduction of any kind into humans or animals is strictly forbidden by law. This product should only be handled by licensed, qualified professionals. This product is not a drug, food, or cosmetic and may not be misbranded, misused or mislabled as a drug, food or cosmetic.
“1mg lyophilized subcutaneous injectables presented in a quantity of 24 vials with 10ml of sterile water for injection for reconstitution.”
“Long-term safety and effects of tesamorelin, a growth hormone-releasing factor analogue, in HIV patients with abdominal fat accumulation Treatment of HIV patients with daily tesamorelin, a growth adverse events during the extension phase was comparable hormone-releasing factor analogue, for 26 weeks resulted with the initial phase. Changes in glucose parameters over in a significant decrease in visceral adipose tissue (VAT) 52 weeks were not clinically significant and similar to those and improvement in lipids. The objective of the 26-week after 26 weeks. The change in VAT was sustained at -18% extension phase was to evaluate long-term safety and effects over 52 weeks of treatment (P < 0.001 versus baseline) as of tesamorelin. HIV patients with central fat accumulation was the change in triglycerides (-51 mg/di, P < 0.001 versus in the context of antiretroviral therapy were randomized to baseline).
Similar sustained beneficial effects were seen for tesamorelin 2 mg (n = 273) or placebo (n = 137) s.c. daily total cholesterol, but high-density lipoprotein decreased for 26 weeks. At week 26, patients originally on minimally over 52 weeks. Upon discontinuation of tesamorelin were rerandomized to 2 mg tesamorelin (T-T tesamorelin, VAT reaccumulated. Treatment with group, n = 154) or placebo (T-P group, n = 50), whereas tesamorelin was generally well tolerated and resulted in patients originally on placebo were switched to sustained decreases in VAT and triglycerides over 52 weeks tesamorelin (P-T group, n = 111). Safety included adverse events and glucose parameters. Tesamorelin was generally without aggravating glucose. Though effects on VAT are sustained during treatment for 52 weeks, these effects do well tolerated. The prevalence of adverse events and serious not last beyond the duration of treatment.
Falutz, Julian & Allas, Soraya & Mamputu, Jean-Claude & Potvin, Diane & Kotler, Donald & Somero, Michael & Berger, Daniel & Brown, Stephen & Richmond, Gary & Fessel, Jeffrey & Turner, Ralph & Grinspoon, Steven. (2008). Long-term safety and effects of tesamorelin, a growth hormone-releasing factor analogue, in HIV patients with abdominal fat accumulation. AIDS (London, England). 22. 1719-28.”
Similar sustained beneficial effects were seen for tesamorelin 2 mg (n = 273) or placebo (n = 137) s.c. daily total cholesterol, but high-density lipoprotein decreased for 26 weeks. At week 26, patients originally on minimally over 52 weeks. Upon discontinuation of tesamorelin were rerandomized to 2 mg tesamorelin (T-T tesamorelin, VAT reaccumulated. Treatment with group, n = 154) or placebo (T-P group, n = 50), whereas tesamorelin was generally well tolerated and resulted in patients originally on placebo were switched to sustained decreases in VAT and triglycerides over 52 weeks tesamorelin (P-T group, n = 111). Safety included adverse events and glucose parameters. Tesamorelin was generally without aggravating glucose. Though effects on VAT are sustained during treatment for 52 weeks, these effects do well tolerated. The prevalence of adverse events and serious not last beyond the duration of treatment.
Falutz, Julian & Allas, Soraya & Mamputu, Jean-Claude & Potvin, Diane & Kotler, Donald & Somero, Michael & Berger, Daniel & Brown, Stephen & Richmond, Gary & Fessel, Jeffrey & Turner, Ralph & Grinspoon, Steven. (2008). Long-term safety and effects of tesamorelin, a growth hormone-releasing factor analogue, in HIV patients with abdominal fat accumulation. AIDS (London, England). 22. 1719-28.”